Synthesis of 2-aminobenzaldehydes by rhodium(iii)-catalyzed C–H amidation of aldehydes with dioxazolones†
Abstract
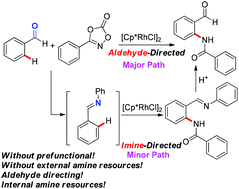
Various aldehydes were directly amidated with dioxazolones to afford 2-aminobenzaldehydes through rhodium(III)-catalyzed CHO-directed C–H activation. Aldehydes as the directing groups can effectively minimize waste formation and pre-functionalization steps. Notably, another pathway through which the traceless imine directing groups are generated by a self-reaction between benzaldehydes and dioxazolones has also been proposed in the catalytic system. Further synthetic application of 2-aminobenzaldehydes can lead to more attractive heterocyclic frameworks.


 Please wait while we load your content...
Please wait while we load your content...