Sulfur mediated propargylic C–H alkylation of alkynes†
Abstract
A transition-metal-free, sulfur mediated propargylic C–H alkylation reaction was realized through a one-pot procedure. The reaction design involves an initial addition between alkynes and triflic anhydride activated sulfoxides, and a subsequent [2,3]-sigmatropic rearrangement of the allenyl sulfur ylides generated under basic conditions to give propargylic C–H alkylation products.
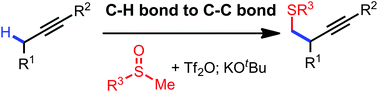
- This article is part of the themed collection: Celebrating the 90th birthday of Professor Lu Xiyan


 Please wait while we load your content...
Please wait while we load your content...