A facile method for the synthesis of trifluoromethylthio-/chloro-homoallylic alcohols from methylenecyclopropanes†
Abstract
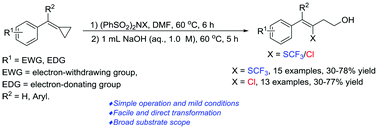
A facile method for the synthesis of trifluoromethylthio-homoallylic alcohols from methylenecyclopropanes (MCPs) has been achieved with the application of (PhSO2)2NSCF3 in N,N-dimethyl formamide (DMF). It has been proved that DMF plays a role both as a solvent and a reagent. Meanwhile, we find that (PhSO2)2NCl is also an active reagent to afford chloro-homoallylic alcohols in the same way as (PhSO2)2NSCF3, and it is very convenient to convert the product to the corresponding amido- or bromo-substituted compounds.


 Please wait while we load your content...
Please wait while we load your content...