The iridium-catalysed reductive coupling reaction of tertiary lactams/amides with isocyanoacetates†
Abstract
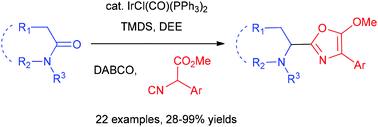
Catalytic C–C bond forming methods for the one-pot transformation of amides into other classes of compounds are highly demanding. In this report, we demonstrate the catalytic reductive addition of isocyanoacetates to tertiary lactams/amides to produce 5-methoxyoxazoles, including N-heterocyclic substituted oxazoles and sterically hindered amino oxazoles that are difficult to obtain by traditional oxazole-forming Ugi-type reactions. This one-pot procedure involves the Ir-catalysed partial reduction of lactams/amides and the sequential chemoselective addition of the isocyanide group in isocyanoacetates.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...