Palladium-catalyzed asymmetric dearomative alkenylation of indoles through a reductive-Heck reaction†
Abstract
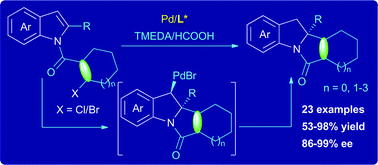
A highly enantioselective intramolecular dearomatizing olefination of indoles has been developed through a Pd-catalyzed reductive Heck reaction. This protocol provides efficient access to optically active tetracyclic indolines bearing C2-alkenylated quaternary stereocenters in good yields with excellent enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...