Total synthesis of teixobactin and its stereoisomers†
Abstract
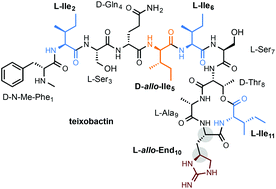
Teixobactin is a depsipeptide natural product with potent antibiotic activity against a range of Gram-positive multi-drug resistant bacteria. Its composition features a unique L-allo-enduracididine (allo-End10), three Iles2/6/11 residues, and one D-allo-Ile5 residue. Intrigued by the potential biological impact of the stereochemistry of these residues, we synthesized teixobactin and three of its stereoisomers carrying L-End10, D-End10 or D-allo-End10. In addition, stereoisomers with D-allo-Ile shuffled to positions 2, 6 or 11 were prepared. Our synthetic strategy uses the combined methods of solution and solid phase peptide synthesis. The solution phase synthesis overcomes the racemization issue for ester coupling between Ile11 and Thr8. The solid phase synthesis improves efficiency and convergence. This method also allows the efficient preparation of fluorescent-labeled analogs of teixobactin.
- This article is part of the themed collection: Organic Chemistry Frontiers HOT articles for 2018


 Please wait while we load your content...
Please wait while we load your content...