The mechanism of ratiometric fluoride sensing and the ESIPT process for 2,6-dibenzothiazolylphenol and its derivative†
Abstract
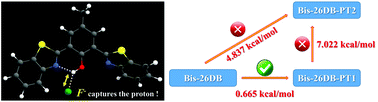
In this work, we explore the excited state intramolecular proton transfer (ESIPT) process and the relevant fluoride-sensing mechanism of two novel chemical systems, 2,6-dibenzothiazolylphenol (26DB) and bis-2,6-dibenzothiazolylphenol (Bis-26DB). The strengthening of the hydrogen bonds in the excited state and the relative charge redistribution promote the ESIPT process. For the molecule Bis-26DB, the S1-state potential barriers reveal an excited state single proton transfer process, rather than an excited state double proton transfer as previously reported from experiment. With the addition of fluoride anion, the ESIPT reaction could be inhibited for both 26DB and Bis-26DB, which determines the fluoride probe response. This work not only elaborates on the ESIPT mechanisms for 26DB and Bis-26DB, but also further reveals the fluoride-sensing mechanism and paves the way for the designing and developing of novel fluorescent probes.


 Please wait while we load your content...
Please wait while we load your content...