Metal Acetylacetonate–Bidentate Ligand Interaction (MABLI) as highly efficient free radical generating systems for polymer synthesis†
Abstract
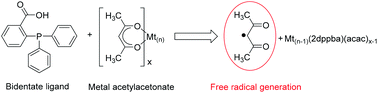
Metal Acetylacetonate–Bidentate Ligand Interaction (MABLI) is presented here as a new chemical mechanism for the highly efficient generation of free radicals for polymer synthesis. This MABLI process involves simultaneous ligand exchange and a change of the metal oxidation degree and is associated with the efficient release of free radicals. In conventional redox two-component radical generating systems, two criteria are required to be efficient: (1) oxidizing agents must exhibit a low bond dissociation energy (BDE) i.e. they are usually unstable (e.g. peroxides) and (2) a small difference must exist between the oxidation potential of the reducing agent and the reduction potential of the oxidation agent. In contrast, here, the criteria for efficient MABLI radical generation were energetic and geometric for both bidentate ligands and metal acetylacetonates. The strength of this approach is to use stable compounds in 2-components free radical initiating systems and to generate carbon centered radicals. Mechanistic investigations demonstrated the formation of new metal adducts by means of high-resolution mass spectroscopy (HR-ESI-MS) as well as UV-vis spectrometry. As a result of its high radical generating rate, the potential of MABLI was illustrated on the methacrylate free radical polymerization under mild conditions (room temperature, in air) and initiated with a small amount of metal acetylacetonate though it opens new perspectives for acac-like additions in organic chemistry.


 Please wait while we load your content...
Please wait while we load your content...