Organic nanoparticles based on Lewis-pair formation: observation of prototropically controlled dual fluorescence†
Abstract
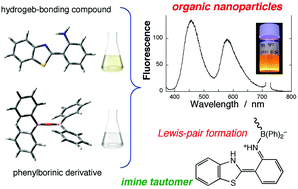
We successfully synthesize fluorescent organic nanoparticles of a Lewis-pair consisting of an amino-type hydrogen-bonding molecule (Lewis base) and a borinate derivative (Lewis acid). 2-(2′-Aminophenyl)benzothiazole (o-ABT) is chosen as the fluorophore. This molecule has a transferable proton in the amino group, but it does not exhibit ESIPT (excited-state intramolecular proton transfer) reaction in solution and thus shows a single normal emission solely from the enamine form. Organic nanoparticles are prepared by the reprecipitation method in which the fluorophore (o-ABT) in conjunction with a Lewis acid (diphenylborinic anhydride; DPBA) dissolved in a good solvent is rapidly injected into water under sonication. Interestingly, the nanoparticles produced exhibit a characteristic dual fluorescence that can be ascribed to the enamine and imine tautomers of o-ABT generated in the ground-state prototropy, which can be revealed by UV-vis absorption and excitation spectroscopy, IR spectroscopy and computational approaches. In the o-ABT/DPBA Lewis-pair nanoparticles, highly Stokes-shifted emission from the imine tautomer is enhanced in comparison with that from the molecularly dissolved state, suggesting that the present nanofabrication methodology based on Lewis acid–base chemistry (or N–B bonding interaction) plays a key role in tuning the fluorescence colour for the new type of organic nanoparticle.


 Please wait while we load your content...
Please wait while we load your content...