Kinetic analysis of tautomer forms of aromatic-urea compounds with acetate ions: solvent effect of excited state intermolecular proton transfer†
Abstract
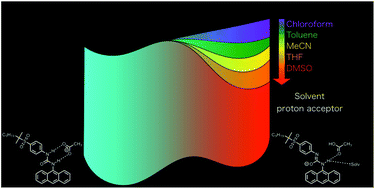
In this paper, we report the solvent effect of excited state intermolecular proton transfer (ESIPT) reactions of urea compounds in the presence of tetrabutylammonium acetate (TBAAc). We prepared anthracene-urea compounds (9An and 2An), a pyrene-urea compound (Py) and an anthracene-diurea compound (9,10An), which have alkylsulfonyl groups to improve their solubility in various organic solvents. We investigated the solvent effects of the ESIPT reaction using absorption, fluorescence, and 1H NMR spectroscopy along with fluorescence decay measurements in dimethyl sulfoxide (DMSO), acetonitrile (MeCN), tetrahydrofuran (THF) and toluene. The tautomer fluorescence of 9An showed remarkable solvent dependence on the spectral red-shift compared with 2An, Py and 9,10An. As a result of the detailed spectroscopic investigations with regard to the solvent including kinetic analysis of the ESIPT for 9An⋯AcO−, we revealed that the energy gap between the normal and tautomer forms in the excited state depended on the hydrogen bond acceptor basicity (β), which is one of the Kamlet–Taft solvent parameters. Finally, we discovered that the tautomer structures of aromatic-urea compounds were stabilized by hydrogen bond interactions.

 Please wait while we load your content...
Please wait while we load your content...