Iodine-mediated regio- and stereoselective iodothiocyanation of alkynes in aqueous ethanol†
Abstract
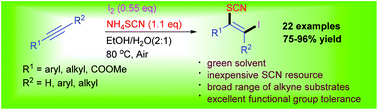
Iodothiocyanation of alkynes with ammonium thiocyanate (NH4SCN) and molecular iodine (I2) has been demonstrated in aqueous ethanol, which enables efficient synthesis of a series of functional β-iodo vinylthiocyanates in good to excellent yields under mild reaction conditions without the need for any protection.


 Please wait while we load your content...
Please wait while we load your content...