Copper-catalyzed synthesis of 2-aminophenyl benzothiazoles: a novel approach†
Abstract
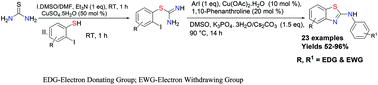
A novel, convenient and efficient protocol for the construction of various 2-aminophenyl benzothiazoles by domino intra- and intermolecular C–N cross-coupling reactions of arylisothioureas with aryl iodides using an inexpensive, air stable and readily available copper catalyst is described. The arylisothioureas were obtained from thiourea via copper promoted desulfurization followed by nucleophilic substitution. In addition, the reactivity of arylhalides and the reaction mechanism have also been studied. The protocol features operational simplicity and a broad substrate scope.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...