Stereoselective synthesis of 1,3-disubstituted dihydroisoquinolines vial-phenylalanine-derived dihydroisoquinoline N-oxides†
Abstract
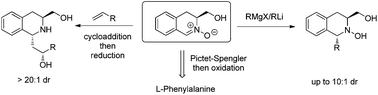
The preparation of chiral pool-derived nitrone 3 and its use in the protecting-group free, stereoselective synthesis of a range of 1,3-disubstituted tetrahydroisoquinolines is described. Grignard reagent additions to nitrone 3 yielded trans-1,3-disubstituted N-hydroxytetrahydroisoquinolines 6 with good levels of selectivity, while 1,3-dipolar cycloadditions to this nitrone provided access to 3-(2-hydroxyalkyl)isoquinolines 12 as single diastereomers.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...