Stereoselective synthesis of sugar-fused (or 1,2-annulated) isochromans and isochromanones by using oxa-Pictet–Spengler reaction†
Abstract
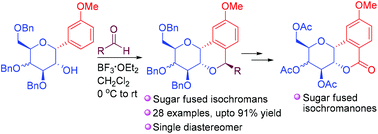
A highly efficient stereoselective synthesis of sugar fused 1,2-annulated isochromans is reported by utilizing the oxa-Pictet–Spengler cyclization reaction. The process is found to be very general as both glucal and galactal derived C2-hydroxy-α-C-aryl glycosides lead to the target molecules in good yields. The utility of this strategy was extended to the synthesis of sugar fused isochromanones, which are bergenin type molecules.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...