Temperature-controlled sequential Suzuki–Miyaura reactions for preparing unsymmetrical terphenyls†
Abstract
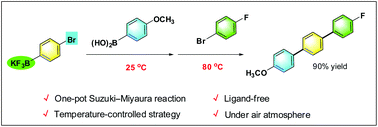
A one-pot protocol of double Suzuki–Miyaura reactions has been developed for the synthesis of unsymmetrical terphenyls. In the absence of a ligand, potassium bromophenyltrifluoroborate reacts with arylboronic acid and then sequentially with a hetero/aryl bromide by controlling the reaction temperature, providing unsymmetrical p- and m-terphenyl compounds in moderate to good overall yields. This protocol provides a convenient and practical approach to unsymmetrical terphenyls under ligand-free and aerobic conditions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...