Chiral β3-isocyanopropionates for multicomponent synthesis of peptides and depsipeptides containing a β-amino acid fragment†
Abstract
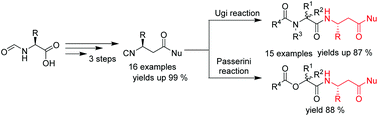
An efficient three-step synthesis of a novel family of enantiomerically pure isocyanides derived from β3-isocyanopropionic acids was elaborated. Easily available N-formylated α-amino acids were used as starting materials towards this aim. The 3-step sequence (Arndt–Eistert reaction–Wolff rearrangement–dehydration) resulted in target isonitriles in good yields (up to 97%). As a result a new family of isocyanides bearing a fragment of β3-amino acids with different functional groups (amides, esters and short peptides) was obtained. It was demonstrated that these new isonitriles can be used in the Ugi and Passerini reactions to prepare short peptides and depsipeptides having a β-amino acid fragment incorporated.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...