o-Nitrobenzyl photoremovable groups with fluorescence uncaging reporting properties†
Abstract
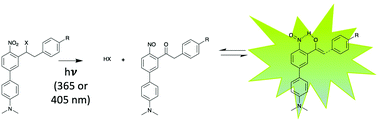
o-Nitrobenzyl (o-NB) derivatives are the most widely applied photoremovable groups for the study of dynamic biological processes. By introducing different substituents to the benzylic position we were able to generate a fluorescence signal upon irradiation. This signal originates from the formation of a nitrosoketone by-product able to achieve a keto–enol tautomerism leading to pi-conjugated α-hydroxystilbene derivatives. These o-NB caging groups can be used to directly monitor the uncaging event by the release of a detectable fluorescent side-product.


 Please wait while we load your content...
Please wait while we load your content...