Strategies for the synthesis of spiropiperidines – a review of the last 10 years
Abstract
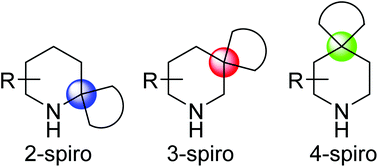
Spiropiperidines have gained in popularity in drug discovery programmes as medicinal chemists explore new areas of three-dimensional chemical space. This review focuses on the methodology used for the construction of 2-, 3- and 4-spiropiperidines, covering the literature from the last 10 years. It classifies the synthesis of each of the types of spiropiperidine by synthetic strategy: the formation of the spiro-ring on a preformed piperidine ring, and the formation of the piperidine ring on a preformed carbo- or heterocyclic ring. While 3- and 4-spiropiperidines are predominantly synthesised for drug discovery projects, 2-spiropiperidines are synthesised en route to natural products. The lack of 2-spiropiperidines in drug discovery is presumably due to limited general procedures for their synthesis.


 Please wait while we load your content...
Please wait while we load your content...