Conformation-guided analogue design identifies potential antimalarial compounds through inhibition of mitochondrial respiration†
Abstract
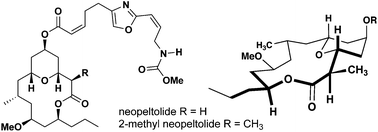
The synthesis of a 2-methyl-substituted analogue of the natural product, neopeltolide, is reported in an effort to analyze the importance of molecular conformation and ligand–target interactions in relation to biological activity. The methyl substitution was incorporated via highly diastereoselective ester enolate alkylation of a late-stage intermediate. Coupling of the oxazole sidechain provided 2-methyl-neopeltolide and synthetic neopeltolide via total synthesis. The substitution was shown to maintain the conformational preferences of its biologically active parent compound through computer modeling and NMR studies. Both compounds were shown to be potential antimalarial compounds through the inhibition of mitochondrial respiration in P. falciparum parasites.


 Please wait while we load your content...
Please wait while we load your content...