Fe-Catalyzed tandem cyclization for the synthesis of 3-nitrofurans from homopropargylic alcohols and Al(NO3)3·9H2O†
Abstract
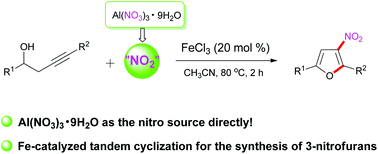
Al(NO3)3·9H2O as a nitro source for the synthesis of 3-nitrofurans from homopropargylic alcohols through Fe-catalyzed tandem cyclization is described. In this transformation, the substituted nitrofurans are obtained through nitration and cyclization. The substrate homopropargylic alcohols with different groups participate smoothly in this process and the desired substituted nitrofurans were obtained in moderate yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...