Rhodium-catalyzed regioselective C8-H amination of quinoline N-oxides with trifluoroacetamide at room temperature†
Abstract
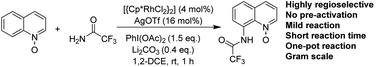
A facile and efficient protocol for rhodium-catalyzed amination of quinoline N-oxides at the C-8 position using simple and commercially available trifluoroacetamide as the amino source has been developed, which proceeds with perfect regioselectivity at room temperature and short reaction times. This catalytic system is highly convenient on a gram scale. The desired products feature diverse functional group tolerance with good to excellent yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...