Synthetic antibody protein mimics of infliximab by molecular scaffolding on novel CycloTriVeratrilene (CTV) derivatives†
Abstract
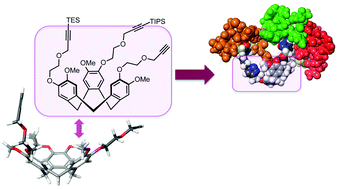
Syntheses of novel semi-orthogonally protected CycloTriVeratrilene (CTV) analogues with enhanced water solubility, that is 3 and 4, derived from the previously described CTV scaffold derivative 2 are described here. These scaffolds 2–4 enabled a sequential introduction of three different complementarity determining region (CDR) mimics via Cu(I)-catalysed azide–alkyne cycloaddition towards medium-sized protein mimics denoted as “synthetic antibodies”. The highly optimised sequential introduction enabled selective attachment of three different CDR mimics in a one-pot fashion. This approach of obtaining synthetic antibodies, demonstrated by the synthesis of paratope mimics of monoclonal antibody infliximab (Remicade®), provided a facile access to a range of (highly) pre-organised molecules bearing three different (cyclic) peptide segments and may find a wide range of applications in the field of protein–protein interaction disruptors as well as in the development of synthetic vaccines or lectin mimics. The prepared synthetic antibodies were tested for their affinity towards tumour necrosis factor alpha using surface plasmon resonance and synthetic antibodies with micromolar affinities were uncovered.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...