Auto-oxidation promoted sp3 C–H arylation of glycine derivatives†
Abstract
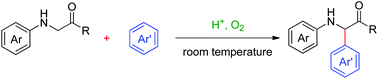
An auto-oxidation promoted sp3 C–H arylation reaction between N-aryl glycine derivatives and electron-rich arenes, leading to the formation of N-aryl α-aryl α-amino acid derivatives, is described. This atom-economical and environmentally benign reaction proceeds smoothly under mild reaction conditions and requires only Brønsted acid and oxygen (balloon). A plausible radical involved mechanism is proposed.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...