A theoretical study on NHC-catalysed enantioselective cycloaddition of ketenes and 3-aroylcoumarins: mechanism and enantioselectivity†
Abstract
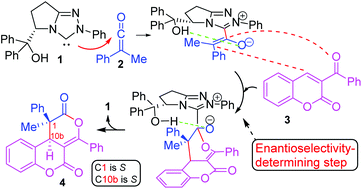
NHC-catalysed enantioselective cycloaddition of ketenes to 3-aroylcoumarins to yield dihydrocoumarin-fused dihydropyranones has been investigated using DFT methods at the B3LYP/6-31G* and MPWB1K/6-311G** computational levels. Two plausible mechanisms have been studied: the “ketene-first” mechanism A and the “coumarin-first” mechanism B. An analysis of the activation Gibbs free energies involved in the two competitive pathways makes it possible to rule out the pathway associated with the “coumarin-first” mechanism B. The first step of the “ketene-first” mechanism A is the formation of zwitterionic intermediate IN1-Zvia a nucleophilic attack of NHC 1 on ketene 2. A [4 + 2] cycloaddition through the nucleophilic attack of enolate IN1-Z on the conjugated double bond of the benzoyl group of coumarin 3, viaTS3-SS-a2 or TS3-RR-a2, yields IN3. Finally, the extrusion of the catalyst through TS5 leads to the final products, either 4-SS or 4-RR. Enantioselectivity observed in the experimental results is determined in the transition states TS3-SS-a2/TS3-RR-a2. In this pathway, the intramolecular hydrogen-bonding between the hydroxyl group of the IN1-Z adduct and the carbonyl oxygen of the original ketene group directs the final stereochemistry throughout the entire process.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...