Synthesis of water-soluble anthracene-appended benzoxaboroles and evaluation of their cis-1,2-diol recognition properties†
Abstract
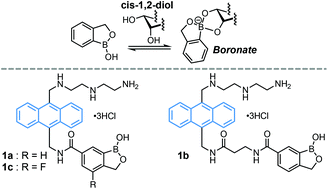
Three series of water-soluble anthracene-appended benzoxaboroles 1a–c were developed; their binding affinity toward cis-1,2-diols was explored by conventional fluorescence titrations to demonstrate the role of benzoxaborole as a general recognition motif of cis-1,2-diols for fluorescent probes. The complex structures of the tetra-coordinated boronate adducts between 1 and the cis-1,2-diols were revealed.


 Please wait while we load your content...
Please wait while we load your content...