Synthesis of fused tricyclic systems by thermal Cope rearrangement of furan-substituted vinyl cyclopropanes†
Abstract
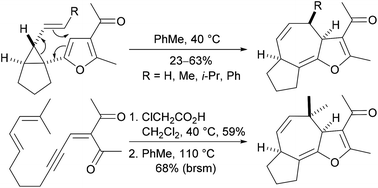
A novel method for the stereoselective construction of hexahydroazuleno[4,5-b]furans from simple precursors has been developed. The route involves the use of our recently developed Brønsted acid catalysed cyclisation reaction of acyclic ynenones to prepare fused 1-furanyl-2-alkenylcyclopropanes that undergo highly stereoselective thermal Cope rearrangement to produce fused tricyclic products. Substrates possessing an E-alkene undergo smooth Cope rearrangement at 40 °C, whereas the corresponding Z-isomers do not react at this temperature. Computational studies have been performed to explain the difference in behaviour of the E- and Z-isomers in the Cope rearrangement reaction. The hexahydroazuleno[4,5-b]furans produced by Cope rearrangement have potential as advanced intermediates for the synthesis of members of the guaianolide family of natural products.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...