Cascade reactions of indigo with oxiranes and aziridines: efficient access to dihydropyrazinodiindoles and spiro-oxazocinodiindoles†
Abstract
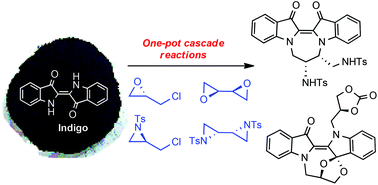
The base-initiated alkylation of the abundant natural dye indigo 1 with ring-strained electrophiles results in the unprecedented, one-pot synthesis of functionalised dihydropyrazino[1,2-a:4,3-a′]diindoles, dihydroepoxy[1,5]oxazocino[5,4-a:3,2-b′]diindoles, and dihydrodiazepino[1,2-a:4,3-a′]diindoles, resulting from intramolecular ring opening-expansion cyclisation processes of their parent oxiranes and aziridines. Regiochemical and stereochemical aspects of the reactions are reported together with integrated mechanistic proposals. This new indigo cascade chemistry should have broad applicability in the synthesis of chemical architectures, not readily-accessible by other means. The three-step synthesis of the useful synthetic precursor (R)-2-(chloromethyl)-1-tosylaziridine 14 is also described. Initial biological activity investigations into these new 2,2′-dindolyl-based heterocyclic derivatives revealed potent, selective antiplasmodial activity in vitro for several isolated structures, with IC50 values as low as 76.6 nM for (±)-8, while demonstrating low human cell toxicity.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...