Total synthesis of the natural HDAC inhibitor Cyl-1†
Abstract
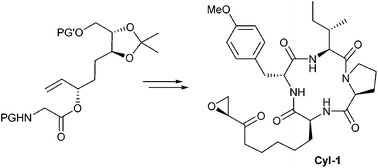
Chelate enolate Claisen rearrangements are powerful reactions for constructing amino acid scaffolds. They generally proceed via chair-like transition states with excellent transfer of stereogenic information. Utilizing this reaction in natural product synthesis gives access to non-proteinogenic amino acids such as (2S,9S)-2-amino-8-oxo-9,10-epoxydecanoic acid (Aoe), the unusual amino acid of a series of histone deacetylase inhibitors (HDACi). Herein the first total synthesis of Cyl-1, a cyclotetrapeptide from Cylindrocladium scoparium, is described.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...