Total synthesis of five natural eremophilane-type sesquiterpenoids†
Abstract
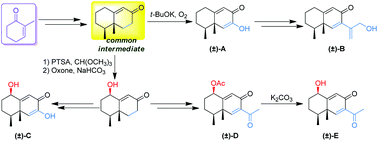
The first total syntheses of five natural eremophilane-type sesquiterpenoids were achieved in 4–12 steps via a common synthetic intermediate. The syntheses feature a double Michael addition, Robinson annulation, α-enolization of an unsaturated ketone, and Pd-catalyzed Suzuki coupling reaction to install the side chain. This synthetic strategy could be easily extended to other eremophilane-type sesquiterpenoids with similar bicyclic skeletons.


 Please wait while we load your content...
Please wait while we load your content...