Negishi cross-couplings in the synthesis of amino acids
Abstract
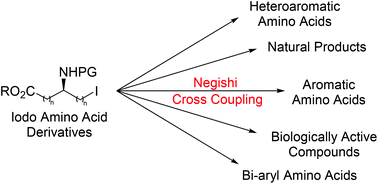
The Negishi cross-coupling is a powerful C–C bond-forming reaction widely utilised in many areas of organic synthesis. This review details the use of Negishi cross-couplings in the synthesis of unnatural amino acids. The application of this reaction in the preparation of aromatic, heteroaromatic, and, complex amino acid derivatives are reviewed and presented herein.


 Please wait while we load your content...
Please wait while we load your content...