Halide exchange and surface modification of metal halide perovskite nanocrystals with alkyltrichlorosilanes†
Abstract
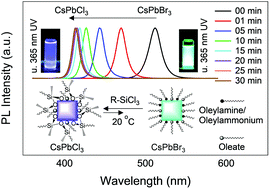
Metal halide perovskite nanocrystals have recently emerged as promising materials for light emitting displays and lasing applications due to their narrow emission wavelengths, high photoluminescence quantum yields, and readily adjustable emission wavelengths. For these metal halide perovskite nanocrystals to be useful in commercial applications, their stability must be increased and the photoluminescence quantum yields of the iodide (red emitting) and chloride (blue emitting) containing derivatives must also be increased. The photoluminescence quantum yields of blue emitting CsPbCl3 nanoparticles lag behind those of green emitting CsPbBr3 nanoparticles, with maximum photoluminescence quantum yields of 1–10% previously reported for CsPbCl3 as compared to 80–100% for CsPbBr3. Herein, we show that alkyltrichlorosilanes (R-SiCl3) can be used as Cl-sources for rapid anion exchange with host CsPbBr3 nanocrystals. This anion exchange reaction is advantageous in that it can be performed at room temperature and results in highly dispersible nanoparticles coated with siloxane shells. CsPbCl3 nanoparticles produced through Cl-exchange with R-SiCl3 show significantly improved long-term stability and high photoluminescence quantum yields of up to 12%. These siloxane coated nanocrystals are even stable in the presence of water, whereas CsPbCl3 nanoparticles synthesized through other routes rapidly degrade in the presence of water.


 Please wait while we load your content...
Please wait while we load your content...
