CeO2−x nanorods with intrinsic urease-like activity†
Abstract
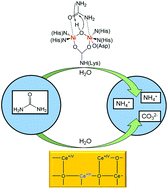
The large-scale production and ecotoxicity of urea make its removal from wastewater a health and environmental challenge. Whereas the industrial removal of urea relies on hydrolysis at elevated temperatures and high pressure, nature solves the urea disposal problem with the enzyme urease under ambient conditions. We show that CeO2−x nanorods (NRs) act as the first and efficient green urease mimic that catalyzes the hydrolysis of urea under ambient conditions with an activity (kcat = 9.58 × 101 s−1) about one order of magnitude lower than that of the native jack bean urease. The surface properties of CeO2−x NRs were probed by varying the Ce4+/Ce3+ ratio through La doping. Although La substitution increased the number of surface defects, the reduced number of Ce4+ sites with higher Lewis acidity led to a slight decrease of their catalytic activity. CeO2−x NRs are stable against pH changes and even to the presence of transition metal ions like Cu2+, one of the strongest urease inhibitors. The low costs and environmental compatibility make CeO2−x NRs a green urease substitute that may be applied in polymer membranes for water processing or filters for the waste water reclamation. The biomimicry approach allows the application of CeO2−x NRs as functional enzyme mimics where the use of native or recombinant enzyme is hampered because of its costs or operational stability.


 Please wait while we load your content...
Please wait while we load your content...