Chemically initiated liquid-like behavior and fabrication of periodic wavy Cu/CuAu nanocables with enhanced catalytic properties†
Abstract
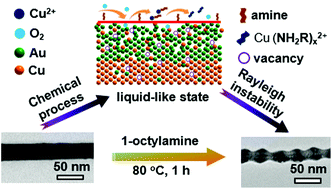
Solid crystalline materials have long range order in their atomic arrangement while liquids have short range order, and the transition between them is usually caused by heat and/or pressure. Herein, we report the finding that chemical processes may play a similar role as heat and initiate liquid-like behavior of crystalline nanomaterials at a temperature far below their melting points. When the straight Cu/CuAu crystalline nanocables are dispersed in organic amine at 80 °C under ambient conditions, the continuous oxidation of Cu atoms on the surface and diffusion of Cu atoms from the core to the surface would break up the long-range ordered arrangement of atoms and lead to the transformation of an anisotropic crystal into an isotropic liquid-like state, which resulted in the evolution of the straight morphology of the nanocables into periodic wavy structures following the Rayleigh instability. It was also demonstrated that periodic wavy Cu@CuAu nanocables exhibit much better catalytic activity than straight Cu@CuAu nanocables towards the reduction of p-nitrophenol into p-aminophenol by NaBH4. Our results not only provide new insights into the transition between a solid crystal and a liquid-like state at the nanoscale, but also facilitate the development of new strategies for the synthesis of functional nanomaterials.


 Please wait while we load your content...
Please wait while we load your content...