Highly selective and sensitive recognition of Zn(ii) by a novel coumarinyl scaffold following spectrofluorometric technique and its application in living cells†
Abstract
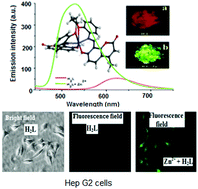
A probe of 8,8′-((1Z,1′Z)-(((propane-1,3-diylbis(oxy))bis(2,1-phenylene))bis(azanylylidene))bis(methanylylidene))bis(7-hydroxy-4-methyl-2H-chromen-2-one) (H2L) was able to recognize Zn2+via its turn-on emission at 510 nm in a MeCN–water (9 : 1, v/v) solution in the presence of a large number of biologically important cations with a very low limit of detection (LOD), namely 11 nM, which meant it was about 3500 times more sensitive than the WHO recommended permissible standard for zinc in drinking water (3 ppm). The probe, H2L, showed solvent polarity sensitive absorption and emission properties and the λmax showed negative solvatochromism. The composition of the complex, [ZnL], was established by a Job's plot, HR-MS data, and single-crystal X-ray diffraction measurements. The selectivity and sensitivity of the probe, H2L, was successfully applied to the detection of intracellular Zn2+ in human hepato-cellular-carcinoma (HepG2) cells. H2L is also applicable for the analysis of Zn2+ ions in tap water.


 Please wait while we load your content...
Please wait while we load your content...