Selective binding of methotrexate to monomeric, dimeric and polymeric cyclodextrins†
Abstract
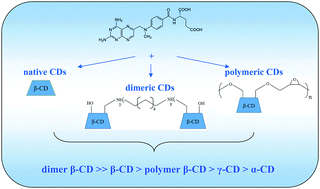
The difference in the complexation affinity of methotrexate (MTX) to the naturally occurring α-, β- and γ-cyclodextrins (CDs) as well as to the novel synthetic dimeric and polymeric β-cyclodextrins was revealed. The effect of CD structure complicity on the complexation with MTX was discussed. It was found that β-CD and dimeric β-CD display a higher binding ability to MTX. Dimeric β-CD acts as a ditopic host forming rather stable 1 : 2 inclusion complexes with MTX. In contrast, the binding of MTX with polymeric β-CD is not sufficient due to the possible steric hindrance. The thermodynamics and binding modes of CDs with MTX were obtained and analyzed. The influence of buffer pH on the complexation process was considered. The binding affinity of β-CD to different ionized forms of MTX was examined using the capillary electrophoresis technique.


 Please wait while we load your content...
Please wait while we load your content...