Comparative studies of the electronic, binding and photophysical properties of a new nona-dentate hemi-cage tripodal HQ pendant trizaza-macrocycle with unfilled, half-filled and completely filled lanthanide ions†
Abstract
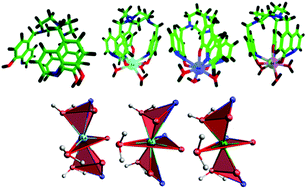
The present paper describes the comparative studies of the electronic, photophysical and binding properties of a new C3-symmetric polydentate ligand, 1,4,7-tris-{(5-methylene-8-hydroxyquinoline)-1,4,7-triazacyclononane} (9N3Me5Ox), and its complexes with 4f0, 4f7, and 4f14 metal ions (La3+, Gd3+, and Lu3+) by experimental and theoretical methods using DFT, TDDFT, TDDFTB, ETS-NOCV, NBO and Ligand Field DFT (LFDFT). The ligand and the complexes were synthesized and characterized through elemental analysis, molar conductance, TGA, FT-IR, FT-NMR, 1H–1H COSY and ESI-mass spectrometry techniques. The spectral data and structural studies revealed the formation of nine-coordinate compounds with the general formula [Ln(9N3Me5Ox)(H2O)3], in which the nonadentate chelator acted as a trinegative hexadentate ligand coordinating to the metal ions through three sets of O,N-donors of 8-hydroxyquinoline groups and three coordinated water molecules. The molecular modeling studies suggest that the metal ion can be easily encapsulated in its central cavity forming hemi-cage complexes without altering the basic metal–ligand coordination sphere. The nature of the bonding between the lanthanide ions and 9N3Me5Ox3−, elucidated by means of the natural bond orbital (NBO), Morokuma–Ziegler energy decomposition analysis (ETS-NOCV) scheme, suggests that the Ln–L bonds are more electrostatic (∼82%) than covalent (∼18%). The covalent character of the complexes increases in the order Lu > La > Gd. The photoluminescence spectral studies of the metal complexes revealed that the observed luminescence of the compounds in the solid state and solution is of different origin. The vibrational, 1H and 13C NMR spectral data obtained from the DFT optimized structures showed good agreement with the experimental results. The excitation and emission behaviours of the ligand and the complexes were established by molecular orbital analysis of the ground state DFT geometry as well as of the excited state optimized geometry using TD-DFT and TD-DFTB orbital analysis, excitation and emission properties.


 Please wait while we load your content...
Please wait while we load your content...