Facile synthesis of nitrogen and sulfur co-doped carbon dots for multiple sensing capacities: alkaline fluorescence enhancement effect, temperature sensing, and selective detection of Fe3+ ions†
Abstract
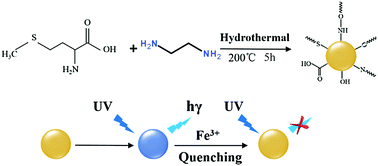
In this work, we prepared nitrogen and sulfur co-doped carbon dots (C-dots) via a one-pot facile hydrothermal method using methionine and ethylenediamine as the precursors. TEM, HRTEM, FTIR and XPS were used to characterize the morphology and surface molecular structures of the N,S co-doped C-dots. The obtained C-dots showed good aqueous solubility and attractive fluorescence properties. The C-dots responded rapidly towards Fe3+ ions, and could be used as a fluorescent probe for Fe3+ ion detection. In addition, the C-dots also exhibited temperature- and pH-sensitive properties, possessing good temperature recoverability and significantly enhanced fluorescence under alkaline conditions. These excellent characteristics promise extensive potential applications for such C-dots in physiological and environmental monitoring.


 Please wait while we load your content...
Please wait while we load your content...