NHC Hg(ii) and Pd(ii) complexes based on 1,8-dihydroxy-9,10-anthraquinone: synthesis, structure and catalysis†
Abstract
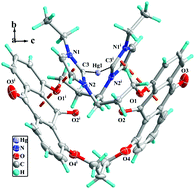
Three bis-azolium salts, 1,8-bis[3′-(N-R-azoliumyl)propoxy]-9,10-anthraquinone hexafluorophosphate L1H2(PF6)2–L2H2(PF6)2 (R = ethyl or picolyl, azoliumyl = imidazoliumyl or benzimidazoliumyl) and 1,3-bis[8′-(3′′-(N-ethyl-imidazoliumyl)propoxy)anthraquinon-l-yloxy]propane hexafluorophosphate (L3H2(PF6)2), as well as their three macrometallocycle N-heterocyclic carbene mercury(II) complexes [L1′HgBr2] (1), [L2Hg](PF6)2 (3) and [L3Hg](PF6)2 (3) were prepared and characterized by 1H NMR and 13C NMR spectroscopy and X-ray crystallography. In complexes 1–3, each macrometallocycle (18-membered macrometallocycle for 1, one 18-membered and two 6-membered macrometallocycles for 2, and 28-membered macrometallocycle for 3) was formed by one biscarbene ligand (L1′ for 1, L2 for 2, and L3 for 3) and one Hg(II) ion. Interestingly, the carbonyl group on the 9-position in complex 1 reacted with two acetonitrile molecules in the presence of the strong base KOtBu through losing one molecule of H2O to form L1′. As a result, two –CH2CN units were introduced to anthraquinone, in which the two –CH2CN units lie on two flanks of the anthraquinone plane. In the crystal packings of 1–3, 2D supramolecular layers and 3D supramolecular frameworks are formed via hydrogen bonds and π⋯π interactions. Additionally, the catalytic activity of the L1–Pd(II) complex in situ in the Suzuki–Miyaura reaction was studied. The results show that this catalyst is efficient in the Suzuki–Miyaura reaction.


 Please wait while we load your content...
Please wait while we load your content...