Cu2+-Induced length change of Ni-based coordination polymer nanorods and research on NiO-based hybrid pseudocapacitor electrodes†
Abstract
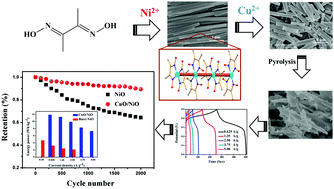
Herein, hybrid Cu/Ni-based hexagonal rods were obtained through a competing reaction of dimethylglyoxime with copper acetate and nickel acetate. Using Cu2+ to intervene the coordination equilibrium between Ni2+ and dimethylglyoxime, the length of the rods was altered; moreover, the coordination environments were investigated by adjusting the concentration of Cu2+ and replacing the solvent. The decomposition products porous NiO and CuO/NiO were directly employed as SC electrodes. The CuO/NiO electrode delivered higher specific capacitance and energy with more excellent energy deliverable ability and better cycling stability than the pristine NiO electrode. After 2000 continuous cycles, the capacitance retention of the as-synthesized CuO/NiO electrode was 87.9%. The maximum specific capacitance and energy delivery efficiency of the CuO/NiO electrode were 344 F g−1 and 93.5%, respectively, at a current density of 0.625 A g−1. The better performance of the hybrid sample was mainly due to higher surface area, lower charge-transfer resistance, and possible synergistic effect. Furthermore, MgO/NiO nanoparticles were synthesized and compared to confirm the existence of a synergistic effect.


 Please wait while we load your content...
Please wait while we load your content...