Strain, switching and fluorescence behavior of a nine-membered cyclic azobenzene†
Abstract
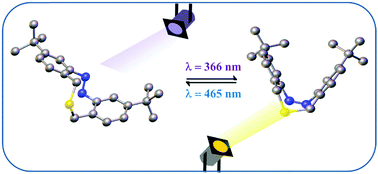
Cyclic azobenzenes with ring strain possess improved photochromic properties in comparison to their acyclic analogs. In this study, we report a nine-membered cyclic azobenzene. This cyclic azobenzene follows the stability of the acyclic systems with the trans isomer as the thermally stable form indicating its flexible structure, yet at the same time, it displays fluorescence properties that are unusual for azobenzenes and require structural rigidity. The properties of the molecule are thus a result of a balance between the flexibility and rigidity of its structure.


 Please wait while we load your content...
Please wait while we load your content...