Ionic liquid-mediated solvothermal synthesis of 4,4′-methylenediphenyl diisocyanate (MDI): an efficient and environment-friendly process†
Abstract
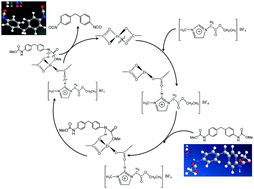
4,4′-Methylenediphenyl diisocyanate (4,4′-MDI) is an immensely important intermediate employed in the manufacturing of polyurethanes. Many synthetic routes have been developed over the decades for the synthesis of 4,4′-MDI compounds on a large scale; these compounds are highly toxic and hazardous in nature. In this study, an environment-friendly route is proposed for the synthesis of 4,4′-MDI using 4,4′-diaminodiphenylmethane and dimethyl carbonate (DMC) as starting materials, which are nontoxic in nature. The synthesis of ionic liquids (ILs) and their utilization in the decomposition reaction are systematically investigated. Imidazole-functionalized ionic liquids were prepared for the synthesis of 4,4′-MDI, and their thermal performances were evaluated by TGA. We found that in comparison with other imidazole-functionalized ionic liquids, 1-ethoxycarbonylmethyl-3-methylimidazolium tetrafluoroborate ([EAmim]BF4) exhibited preferable thermal activity for the decomposition of 4,4′-methylenediphenyl dimethylcarbamate (4,4′-MDC). Moreover, these ILs were more effective when they were combined with zinc as a catalyst, which enhanced the decomposition of MDC. Under optimal conditions, the yield of MDI compared to that of Zn(OAc)2–[EAmim]BF4 catalyst increased up to 96%. The mechanism of the enhanced performance of ionic liquids by catalytic activity of zinc acetate was also investigated.


 Please wait while we load your content...
Please wait while we load your content...