Two isostructural linear coordination polymers: the size of the metal ion impacts the electrical conductivity†
Abstract
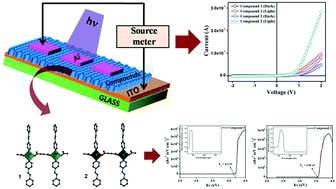
Two new one-dimensional coordination polymers (1D CPs) {[Zn(adc)(4-spy)2(H2O)2]}n (1), and {[Cd(adc)(4-spy)2(H2O)2]}n (2) (H2adc = acetylenedicarboxylic acid and 4-spy = 4-styrylpyridine), have been synthesized and well characterized. Single crystal X-ray diffraction data exhibit that compounds 1 and 2 are isostructural and undergo hydrogen bonding and C–H⋯π interactions to construct 3D supramolecular architectures. Electrical characterization reveals that both compounds show substantive electrical conductivity and exhibit Schottky diode nature. However, compound 2 has a higher mobility and higher conductivity compared to 1. The estimated values of effective carrier mobility, transit time, carrier concentration and diffusion length demonstrate that the charge transport properties have been improved for 2. Therefore, compound 2 has better performance in the fabrication of electronic devices.


 Please wait while we load your content...
Please wait while we load your content...