A new class of triphenylamine-based novel sensitizers for DSSCs: a comparative study of three different anchoring groups†
Abstract
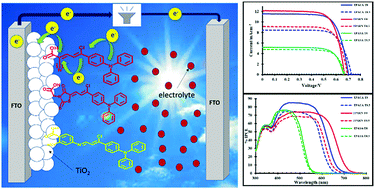
In this paper, we report a new class of triphenylamine-based sensitizers with different anchoring groups to rationalize the effects of the anchoring group on the power conversion efficiencies (PCE) of these sensitizers in dye sensitized solar cells (DSSCs). The photophysical properties of the TPACA, TPARN and TPASA sensitizers were recorded in acetonitrile solvent. DSSCs based on TiO2 photoanodes were fabricated using these dyes. A TPACA chromophore-based DSSC containing triphenylamine as a donor group and cyanoacetic acid as an anchoring group connected by a π-linkage produced a maximum power conversion efficiency of 5.8%, a short circuit current Jsc = 11.92 mA cm−2, an open circuit voltage Voc = 0.702 V and a fill factor FF = 0.716. This enhanced efficiency of the TPACA chromophore-based DSSC is attributed to the presence of a strong electron withdrawing cyanoacetic acid anchoring group. The electron transfer kinetics was investigated by transient absorption spectroscopy (TAS). All three chromophores, TPACA, TPARN, and TPASA, were extensively studied using density functional theory (DFT) and time dependent density functional theory (TD-DFT) to correlate the effects of the anchoring modifications with their absorptions and photoconversion efficiencies.


 Please wait while we load your content...
Please wait while we load your content...