High performance direct hydrazine–hydrogen peroxide fuel cell using reduced graphene oxide supported Ni@M (M = Pt, Pd, Ru) nanoparticles as novel anodic electrocatalysts
Abstract
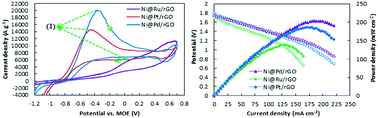
In this study, different core@shell nanoparticles such as Ni@Pt, Ni@Pd and Ni@Ru on reduced graphene oxide (rGO) were synthesized using a two-step successive reduction method with sodium borohydride and ethylene glycol as reducing agents. The obtained electrocatalysts were characterized using field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HR-TEM) and X-ray photoelectron spectroscopy (XPS). The hydrazine oxidation on Ni@Pt/rGO, Ni@Pd/rGO and Ni@Ru/rGO electrocatalysts was studied in a three electrode set-up. The results showed that the hydrazine oxidation current density on Ni@Pd/rGO (19 722 A g−1) is 1.31 and 3.21 times higher than those of Ni@Pt/rGO (15 030 A g−1) and Ni@Ru/rGO (6140 A g−1), respectively. The activation energy for hydrazine oxidation on Ni@Pd/rGO (7.1 kJ mol−1) is lower than that on Ni@Pt/rGO (14.2 kJ mol−1) and Ni@Ru/rGO (28.4 kJ mol−1). Also, the influence of Ni@Pt/rGO, Ni@Pd/rGO and Ni@Ru/rGO as an anodic electrocatalyst on the performance of direct hydrazine–hydrogen peroxide fuel cells (DHHPFCs) was investigated. The single fuel cell results showed that Ni@Pd/rGO resulted in an improvement in power density (204.79 mW cm−2) equal to 10.03% and 47.32% with respect to Ni@Pt/rGO (186.12 mW cm−2) and Ni@Ru/rGO (139.01 mW cm−2), respectively.


 Please wait while we load your content...
Please wait while we load your content...