Aqueous protocol for allylic arylation of cinnamyl acetates with sodium tetraphenylborate using a Bedford-type palladacycle catalyst†
Abstract
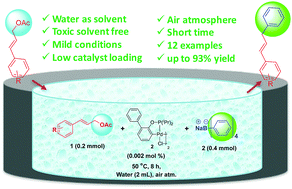
Allylic arylation of cinnamyl acetates with sodium tetraphenylborate using 0.002 mol% of a Bedford-type palladacycle catalyst is described. The developed methodology is applicable for a wide range of cinnamyl acetates furnishing excellent yields of up to 93%. Notably all reactions proceed smoothly under mild reaction conditions in water under an air atmosphere.


 Please wait while we load your content...
Please wait while we load your content...