Boron–boron, carbon–carbon and nitrogen–nitrogen bonding in N-heterocyclic carbenes and their diazaboryl and triazole analogues: Wanzlick equilibrium revisited†
Abstract
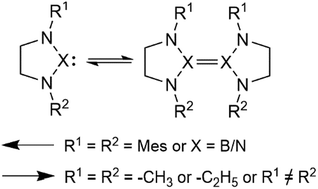
A series of monomers and dimers of N-heterocyclic carbenes and their diazaboryl and triazole analogues were investigated using the M06-2X and M06 density functional methods as well as using a DLPNO-CCSD(T) approach. We show that asymmetric carbenes bearing a benzyl and a mesityl moiety are predicted to form stable dimers due to favorable off-centre parallel stacking interactions. We also show that the double bond of the imidazole core destabilizes carbene dimers, shifting the Wanzlick equilibrium towards monomers. We predict that the methyl-, ethyl- and mesityl-substituted diazaboroles are more stable as monomers, irrespective of the presence of the double-bond in their structure, as are triazoles. Additionally, we demonstrate that the HOMO–LUMO gap difference between the monomer and the dimer, which is relatively easy to obtain, is a good indicator of the relative stability of the dimer versus the monomer for carbenes.


 Please wait while we load your content...
Please wait while we load your content...