Structures and single crystal to single crystal transformations of cadmium frameworks using a flexible tripodal ligand†
Abstract
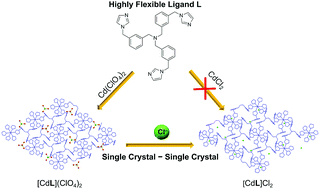
A highly flexible tris-imidazole ligand N(CH2-m-C6H4-CH2-imidazole)3 (L) constructed using a tris(xylyl) backbone was synthesized. Its reactions with three cadmium(II) salts gave various Cd(II) coordination polymers, namely [CdL2](ClO4)2 (1), [CdLCl2] (2), and [CdL(OAc)2] (3). X-ray studies revealed that 1 crystallized in the trigonal space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) with high symmetry, while 2 and 3 crystallized in lower symmetrical space groups due to Cd–anion coordination. The flexibility of ligand L was evidenced in these structures, showing different conformations of L upon coordination and crystallization. In addition, complex [CdL2]Cl2 (4) was obtained by the anion exchange of 1 with sodium chloride via a single crystal to single crystal transformation. Similar to 1, complex 4 also crystallized in the trigonal space group R
with high symmetry, while 2 and 3 crystallized in lower symmetrical space groups due to Cd–anion coordination. The flexibility of ligand L was evidenced in these structures, showing different conformations of L upon coordination and crystallization. In addition, complex [CdL2]Cl2 (4) was obtained by the anion exchange of 1 with sodium chloride via a single crystal to single crystal transformation. Similar to 1, complex 4 also crystallized in the trigonal space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) with a = 13.0945(3), b = 37.4353(10), and V = 5559.2(2). Importantly, this single crystal to single crystal transformation is crucial to afford this Cd(II) coordination polymer with unbound Cl− anions, as the direct reaction of L and CdCl2 gives complex 2 with Cd-bound Cl−. This work shows the importance of flexible ligands in constructing various coordination polymer structures through the regulation of anions.
with a = 13.0945(3), b = 37.4353(10), and V = 5559.2(2). Importantly, this single crystal to single crystal transformation is crucial to afford this Cd(II) coordination polymer with unbound Cl− anions, as the direct reaction of L and CdCl2 gives complex 2 with Cd-bound Cl−. This work shows the importance of flexible ligands in constructing various coordination polymer structures through the regulation of anions.


 Please wait while we load your content...
Please wait while we load your content...