Design, synthesis, anti-proliferative evaluation and docking studies of 1H-1,2,3-triazole tethered ospemifene–isatin conjugates as selective estrogen receptor modulators†
Abstract
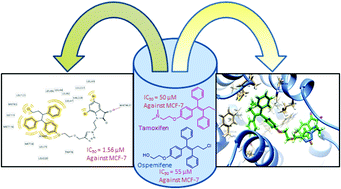
A library of 1H-1,2,3-triazole-tethered ospemifene–isatin and ospemifene–spiroisatin conjugates have been synthesized and evaluated for their anti-proliferative activities against MCF-7 and MDA-MB-231 cell lines. The evaluation studies revealed that compound 11j was the most potent with an IC50 value of 1.56 μM against the MCF-7 cell line. Compounds 11k and 11l also displayed a similar trend, with several-fold lower effective concentrations in ER+ cells than in ER− cells. SAR studies revealed that conjugates having a bromo-substituent at the C-5 and C-7 positions of the isatin ring with ethyl/propyl as the spacer were observed to be active with the most potent compound being ∼30 times more potent than Tamoxifen against the MCF-7 cell line. The evaluation results were further supported by docking studies and the stronger binding affinity of the synthesized conjugates was attributed to their greater structural bulk and greater occupation of the ERα active site.


 Please wait while we load your content...
Please wait while we load your content...