High affinity interactions of Pb2+ with synaptotagmin I†
Abstract
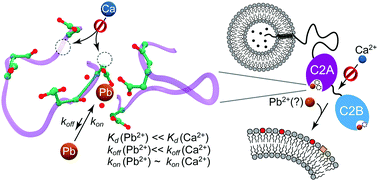
Lead (Pb) is a potent neurotoxin that disrupts synaptic neurotransmission. We report that Synaptotagmin I (SytI), a key regulator of Ca2+-evoked neurotransmitter release, has two high-affinity Pb2+ binding sites that belong to its cytosolic C2A and C2B domains. The crystal structures of Pb2+-complexed C2 domains revealed that protein-bound Pb2+ ions have holodirected coordination geometries and all-oxygen coordination spheres. The on-rate constants of Pb2+ binding to the C2 domains of SytI are comparable to those of Ca2+ and are diffusion-limited. In contrast, the off-rate constants are at least two orders of magnitude smaller, indicating that Pb2+ can serve as both a thermodynamic and kinetic trap for the C2 domains. We demonstrate, using NMR spectroscopy, that population of these sites by Pb2+ ions inhibits further Ca2+ binding despite the existing coordination vacancies. Our work offers a unique insight into the bioinorganic chemistry of Pb(II) and suggests a mechanism by which low concentrations of Pb2+ ions can interfere with the Ca2+-dependent function of SytI in the cell.
- This article is part of the themed collection: Metallomics Recent HOT articles


 Please wait while we load your content...
Please wait while we load your content...
