Structural motif, topi and its role in protein function and fibrillation†
Abstract
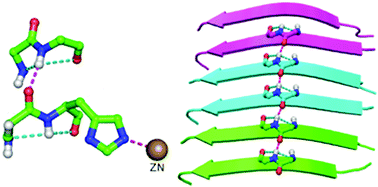
A protein chain is arranged into regions in which the backbone is organized into regular patterns (of conformation and hydrogen bonding) to form the most common secondary structures, α-helix and β-sheet, which are interspersed by turns and more irregular loop regions. A structural motif, topi, is discussed in which a pair of 2-residue segments, each containing hydrogen-bonded five-membered fused-ring motifs, distant in sequence are linked to each other by a hydrogen bond. Though a small motif, it appears to be important in the context of local folding patterns of proteins and occurs near protein active sites. The motif shows quite significant residue preference, and a Cys (or Ser) occupying the second position may further stabilize the motif by forming an additional hydrogen bond across it. Remarkably, topi is found within disease causing misfolded proteins, such as the fibrilled form of Aβ42, and also across the interface between two protein chains. This motif may be an important component of fibrillation and useful for modeling loop regions.


 Please wait while we load your content...
Please wait while we load your content...